Why is the Agency Abrogating its Responsibility?
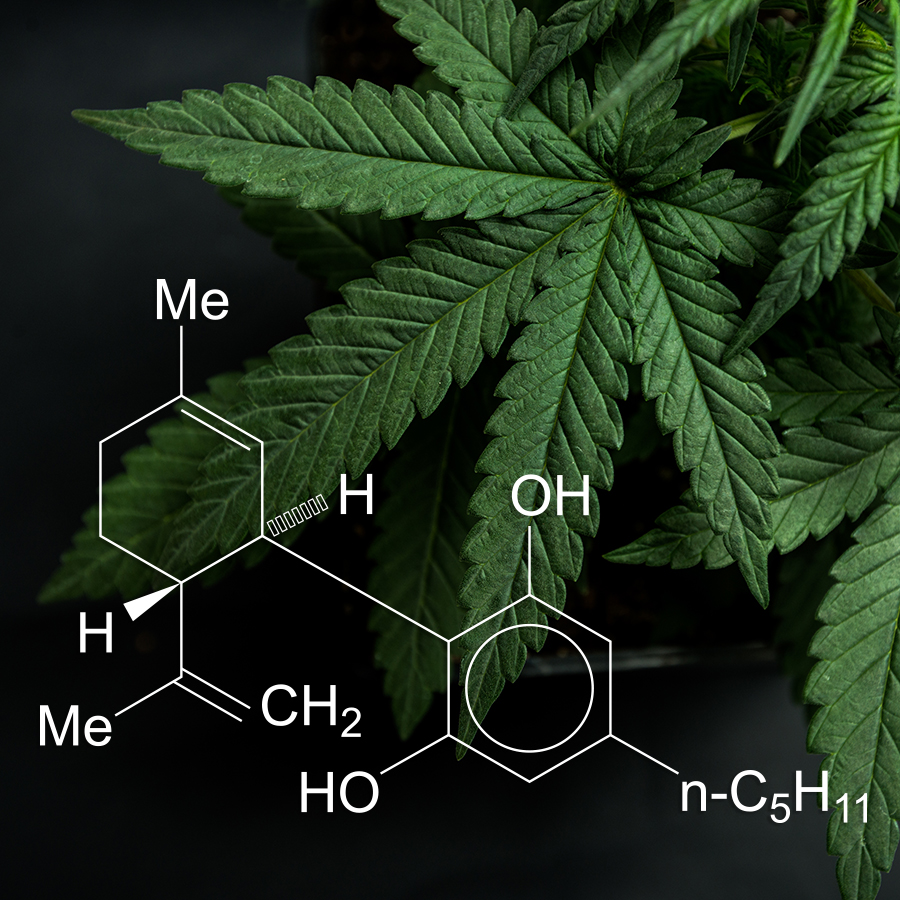
Cannabis is rapidly gaining traction in the United States. It’s a phenomenon being driven by its reputation as a wonder plant capable of improving the wellbeing of people suffering from a variety of ailments. This dramatic increase in demand has spawned a proliferation of companies producing a broad range of consumer goods containing cannabis, including CBD-rich products. But to date, the Food and Drug Administration (FDA) has largely resisted doing any meaningful and logical regulation of this market.
The reluctance to get involved is a curious development, because this is the agency’s jurisdiction. Formed in 1906, the FDA is a consumer protection agency within the U.S. Department of Health and Human Services that was formed with a mandate to safeguard the health of Americans. Its responsibilities are ensuring the safety of the country’s food supply, cosmetics, dietary supplements and tobacco products, as well as human and veterinary drugs, vaccines, medical devices, and other biological products for human use. Cannabis products are being manufactured and consumed that fall into several of these categories.
The situation as it stands now for Americans seeking medicinal relief from health conditions is dire. They are frequently turning to opioids, a dangerous and destructive option. According to the Centers for Disease Control and Prevention (CDC), there were more than 400,000 drug overdose deaths in the United States from 1999 to 2017 that involved opioids. That includes prescription drugs and illegal opioids such as heroin and illicitly manufactured fentanyl. Opioids are involved in drug overdose mortalities six times as often as they used to be, with 130 Americans dying each day from an opioid overdose.
The official reasons for the FDA’s reluctance to show leadership when it comes to CBD were outlined in a 5½-page press release issued on April 2, 2019. The statement, issued by FDA Commissioner Steve Gottlieb, reads more like a set of debatable justifications and flimsy excuses than a convincing stand on the agency’s response to a blossoming industry. Gottlieb talks about forming exploratory groups, holding public hearings, updating the FDA website’s questions and answers section, and sending warning letters to companies marketing CBD products making health claims. All of this bureaucratic mumbo jumbo smacks of lip service and stall tactics designed to protect the interests of Big Pharma.
Commissioner Gottlieb writes, “We’re interested in how the incentives for, and the feasibility of, drug development with CBD and other cannabis-derived compounds would be affected if the commercial availability of products with these compounds, such as foods and dietary supplements, were to become significantly more widespread. We don’t want companies to forgo research that might support approval through the FDA’s drug review process, which could potentially lead to important safe and effective therapies.”
But in case Commissioner Gottlieb hasn’t noticed, the horse has already left the barn so far as CBD is concerned.
So why is the FDA dragging its heels when it comes to showing leadership to help consumers access a plant with promising health benefits, a plant that was first cultivated in the United States as far back as the early 1600s? I believe this reluctance can be summed up in three simple words:
Follow the money.
Back in the 1990s, laws changed to allow the pharmaceutical industry to fund a greater portion of the FDA’s budget. In 2019, the agency spent $1.881 billion on prescription drug oversight. Congress designated $663 million to that budget, and drug companies provided $1.218 billion. With the vast majority of the funding—65%—coming from the industry the FDA is tasked with regulating, how can the FDA possibly be unbiased? This financial support buys the pharmaceutical industry significant influence, a dynamic otherwise known as “regulatory capture.” Given the way the system is currently set up, we can’t expect the FDA to bite the hand that feeds it.
Paul Brown is the manager of government relations for the National Center for Health Research, a think tank that receives no money from the drug industry. He spoke about this conflict of interest at an FDA forum in August 2016. “Have user fees changed FDA’s priorities?” he asked. “Is the FDA now treating industry as a customer that it needs to please instead of acting as a regulator to ensure the public health?”
The financial ties run deep. An analysis done by Science magazine, a respected peer-reviewed academic journal published by the American Association for the Advancement of Science, revealed just how far the money connections go. To gather their information, they used numerous sources including publicly available physician disclosures, records from the Centers for Medicare and Medicaid Services, and data on research funding. The findings from the Science study were eye opening. Physician panel members who advised the FDA on whether to approve particular drugs earned large sums of money. The 16 top-earning advisors received more than $300,000 each for their services. And the FDA’s safeguards against potential conflicts of interest don’t take into account future financial benefits. The same top advisors received another $24 million in personal payments or research support following their term on the panel, and 93% of that money came from the manufacturers of drugs they had previously reviewed, or from their competitors. That is a colossal conflict of interest.
To understand why the FDA would resist the approval of cannabis as a supplement or food additive, we need to understand Big Pharma’s interests. The pharmaceutical industry can’t patent full-plant medicine, so instead it has deconstructed the cannabis plant, breaking it down into isolates in an attempt to enhance Mother Nature and secure patents on the improvements. But this process destroys the benefits and eliminates the coveted “Entourage Effect,” a reality that has frustrated Big Pharma’s patent objectives. Quite simply, the best cannabis medicine is made from the whole plant, and that isn’t patentable. To receive a valid, defensible patent, the applicant must meet particular and substantive conditions.
As it stands now, the American public is facing a sea of CBD in a confusing marketplace with little guidance being offered from government regulators. Desperate to find relief from a wide range of health issues, people are at risk of consuming products containing a slew of dangerous ingredients including heavy metals, solvents, pesticides, and microbial contaminants. It’s not cannabis that presents the danger here. It’s a government captured by Big Pharma, the same companies that manufactured the opioids that have caused the health crisis of addiction and innumerable overdose deaths we’re currently facing. The pharmaceutical industry stands to lose if cannabis gains the foothold it deserves in the wellness market.
Written by Dr. Michael Steward, Chief Medical Officer- https://endourage.com/affiliate-partner/mjcom